Synapses and neural circuits in behaviour
Team Leader : Christophe Mulle and Mario Carta

Christophe Mulle is a research director (DRCE) at the CNRS. He's a cellular neurobiologist with expertise in glutamate receptors, electrophysiology of synaptic transmission and neuronal circuits. After being trained in the labs of Jean-Pierre Changeux at the Pasteur Institute and Steve Heinemann (Salk Institute, San Diego), he created a CNRS laboratory in Bordeaux (1995). His research focuses on the mechanisms underlying the plasticity of synaptic properties and neural circuits in the hippocampus, in the context of episodic-like memory encoding. Great efforts are made to implement these questions at an integrated level in the mouse by developing methods for interrogating the connectivity and function of local circuits in vivo in behavioural conditions. These studies address physiological mechanisms as well as synaptic dysfunction in the context of Alzheimer's disease. In parallel, he has engaged into a translational project targeting ectopic KARs aberrantly expressed in the hippocampus, in temporal lobe epilepsy, in close collaboration with Valérie Crépel (Inserm, Marseille). In 2016, he was elected member of the Academia Europeae. In 2014, he has received the Grand Prix Lamonica de Neurologie of the French Academy of Science.
Mario Carta (CRCN-CNRS, University of Bordeaux)is a neurobiologist with expertise in electrophysiology of synaptic transmission and plasticity and in cortical processing in vitro and in vivo. The general aim of his research is to understand how synapses and neuronal circuits function and how they are regulated in various physiological or pathological states. He was trained as a slice electrophysiologist in the Valenzuela lab (University of New Mexico, USA) where he studied how ethanol, neurosteroids and various pharmacological compounds modulate neuronal and synaptic plasticity and circuit functions in the hippocampus and cerebellum. He worked as a post-doc in the laboratory of Christophe Mulle (University of Bordeaux) to tackle molecular and cell biological aspects of synaptic physiology and plasticity. In 2013, he was recruited as a junior researcher at the CNRS. From 2017-2021, he joined the laboratory of James Poulet (MDC, Berlin) where he investigated in vivo cortical circuits during sensory stimulation and behavior, focusing on the role of the mouse insular cortex in thermosensory perception. He learned how to use cutting-edge approaches such as wide field, 2-photon calcium imaging and electrophysiology in anesthetized and awake behaving animals. He returned to Bordeaux in 2022, where he now investigates the synaptic mechanisms underlying gustatory processing in the mouse cortex.
General objective

The general aim of the team is to understand how cortical synaptic circuits process and encode sensory and contextual information . We investigate in parallel the CA3 region of the hippocampus and the gustatory cortex (GC). The main strength and originality of our research lies in the systematic back-and-forth interrogation of synapses and circuits ex vivo at a molecular and cellular level, and in vivo in the context of intact neural circuits in behaving mice.
Processing and storage of information depend on multiple mechanisms, from activity-dependent plasticity processes with distinct temporal dynamics to integration of different inputs along the dendritic arborization. We will focus on the mechanisms and integrated consequences of short-term presynaptic plasticity at identified cortical synapses. Presynaptic plasticity occurs within a short- term (seconds to minutes) and long-term (up to hours) time frame at thalamo-cortical and cortico-thalamic synapses, as well as at mossy fiber synapses in CA3. Presynaptic plasticity is postulated to be powerful in controlling synaptic cortical circuits. However direct evidence for a role in controlling sensory and contextual information processing is lacking.
Synaptic alteration is a strong predictor of cognitive decline in Alzheimer’s disease (AD). We propose to explore presynaptic failure as a physiopathological determinant of AD pathology. We will use genetic tools and electrophysiology in mice to better understand the physiological and pathological role of the Amyloid Precursor Protein (APP), a central protein in AD, which is abundantly expressed in presynaptic compartments. In parallel we will implement innovative gene targeting methods applied to human organotypic cortical slices in combination with electrophysiology and high-resolution imaging.
The team has engaged into a translational project targeting aberrantly expressed KARs in temporal lobe epilepsy, in collaboration with Valérie Crépel (Inserm, Marseille). Christophe Mulle is one of the scientific founders of Corlieve Therapeutics (www.corlieve.com), a biotech company which aims at finding new therapies to cure intractable temporal lobe epilepsy. The methodology includes work on acute and organotypic human hippocampal and cortical slices from patients with intractable TLE.
Expertise
News
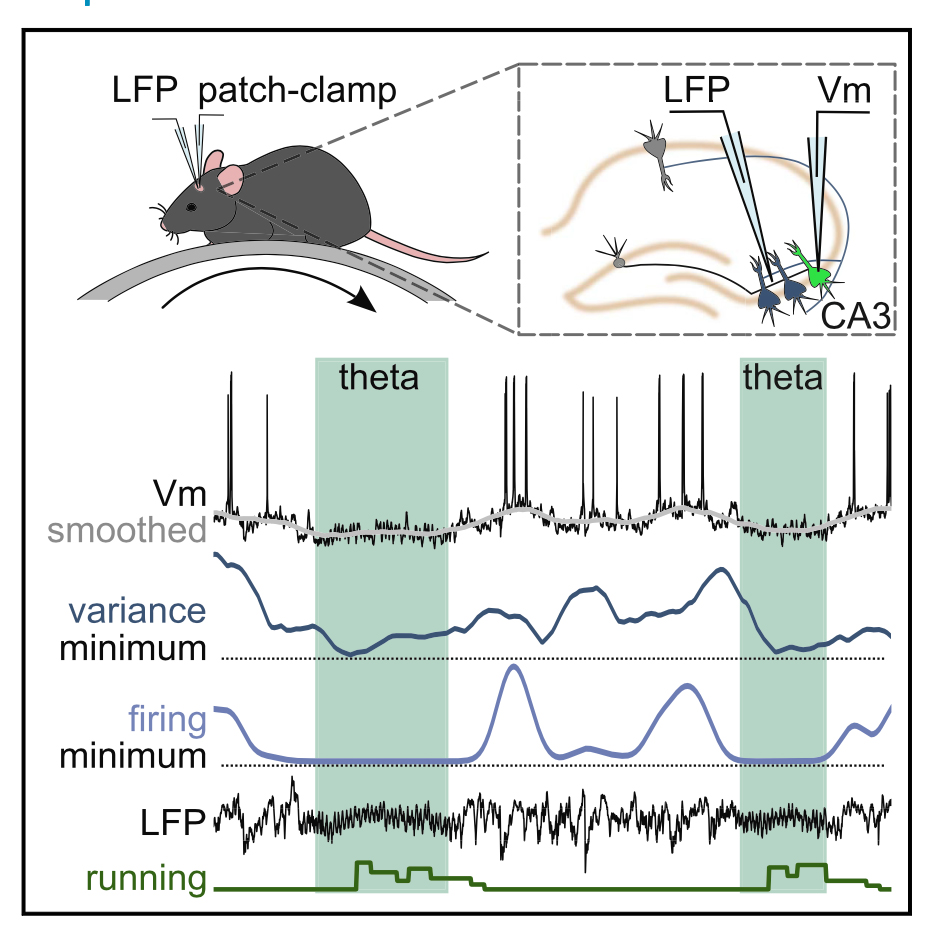
The relationship between brain state and membrane potential in CA3 pyramidal cells
Wakefulness is comprised of distinct brain states correlated with different behaviors and stages of memory. It is hypothesized that memory encoding and recall are more prominent during active behaviors, while memory consolidation is more prominent during rest. The hippocampal region of the brain is involved in all these stages of memory during their respective brain states. For this brain circuit to perform these different computations at different times, it has been hypothesized that the membrane potential of individual neurons must change in a brain state-dependent manner. We sought to test this hypothesis by recording membrane potential from individual CA3 hippocampal pyramidal cells in awake mice during active and restful behaviors. When animals are actively moving, the hippocampal local field potential displays a 4-12 Hz oscillation known as theta. We found that, consistent with the hypothesis, CA3 pyramidal cells underwent consistent changes in membrane potential when theta was present in the local field potential. Specifically, these cells hyperpolarized, decreased firing, and had low membrane potential variance, all of which are consistent with increased inhibition. This sustained, coherent suppression of CA3 pyramidal cells during theta likely changes the circuit dynamics within the hippocampus, contributing to a functional switch that might underlie the ability of the hippocampus to participate in memory encoding during theta.
- Cell Reports 2020 - doi: 10.1016/j.celrep.2020.107868
- Authors and contacts: Meryl Malezieux, Ashley L Kees and Christophe Mulle
+ Cf Bordeaux Neurocampus website here
Corlieve Therapeutics, co-funded by Christophe Mulle acquired by the biotech uniQure:
Corlieve Therapeutics co-funded by Christophe Mulle and Valérie Crépel (Inserm Marseille) acquired by the biotech uniQure. The acquisition of Corlieve Therapeutics by uniQure amounts to 250 million euros, of which the first initial settlement of 46.3 million euros finalizes the transaction.
https://www.bordeaux-neurocampus.fr/en/uniqure-to-acquire-corlieve-therapeutics/
MORE
Awarded 2022 Prize for Innovation of the Académie des Sciences et Belles Lettres de Bordeaux

Valérie Crépel (INSERM) at the University of Aix-Marseille and Christophe Mulle (CNRS) at the Interdisciplinary Institute for Neuroscience at the University of Bordeaux have been awarded the 2022 Prize for Innovation of the Académie des Sciences et Belles Lettres de Bordeaux.
Valérie Crépel and Christophe Mulle are very high-level researchers interested in the physiology and pathology of the hippocampus circuits, a region of the brain located in the temporal lobes, which is the seat of memory and learning.
Their highly innovative research focuses on the therapeutic approach to the treatment of temporal lobe epilepsy. This pathology affects 1.3 million people in Europe and the United States, including 800,000 patients who are resistant to known treatments. Christophe Mulle and Valérie Crépel propose gene therapy as an alternative to surgery and its drawbacks. Their therapeutic proposal is developed within Corlieve Therapeutics: it uses microRNA technology, nucleic acids capable of acting at the level of messenger RNA to selectively reduce aberrant effects in the hippocampus of patients.
Christophe Mulle receives the Prix Desmarest
Le Prix Desmarest of the Pierre Deniker Foundation, aims to fund basic research projects in the field of Alzheimer's disease and neurodegenerative diseases, with a total endowment of €100,000. The Pierre Deniker Foundation supports mental health research programs, and raises public awareness of mental disorders.
For this third edition, the Prix Desmarest was awarded to Christophe Mulle, CNRS research director. The project, which will be co-headed by Thierry Amedee, aims to investigate the morpho-functional relationships between microglia and synapses near amyloid plaques in an animal model of Alzheimer's disease. Christophe Mulle was awarded the prize on January 20th at the Encephale Congress (Paris) by Annick Desmarest and by Chantal Henry, scientific director of the Pierre Deniker Foundation.
Learn more about: Christophe Mulle and Thierry Amedee
Do you want to know more? Nolwenn Cloarec, communication officer: nolwenn.cloarec@u-bordeaux.fr

Portrait of Mario Carta, IINS team leader and CNRS researcher
Read the portrait of Mario Carta in English and French
Mario Carta is a native of Sardegna, Italy. He is a neurobiologist with a long-standing interest in synaptic transmission and plasticity in cortical circuits. Recently, he has been focus his research to the study of how cortical circuits encode sensory information. Thus, together with Mikkel Verstergard a postdoc from the laboratory of James Poulet (MDC, Berlin), they published a study in Nature which reports the discovery of a ‘thermal cortex’ located in a posterior region of the insular cortex. This cortical region is involved in the perception of warm and cool.
You recently published an article in Nature. Can you tell us more about it?
"The cortex receive and compute information from the external world to guide our behaviour. Where and how thermal stimuli are processed in the cortex was not known. Together with Mikkel Verstergard, we wanted to understand how non-painful temperatures are encoded at the single cell level in the mammalian cortex. Therefore, we have developed a preparation to optically access the mouse insular cortex. For this purpose, we used large-scale imaging approaches such as wide-field and two-photon calcium imaging in awake behaving mice to record neuronal activity. Finally, in order to confirm the role of the insular cortex in temperature perception, we performed optogenetic manipulations. Thus, our study demonstrated that cooling and warming are coded differently in the mammalian cortex. This highlight the complexity of temperature perception in the brain!"
What is your background?
"I started by studying biology at the University of Cagliari in Italy. Then I obtained the equivalent of a master’s degree in Valenzuela’s laboratory in Albuquerque, USA. Finally, I returned to Italy and obtained a PhD in neuroscience at the
University of Cagliari. [...] Throughout my career, I have a strong background in slice electrophysiology and I have recently shifted my attention to the investigation of neuronal circuits in behaving animals."
Why did you choose neuroscience?
"During my university studies, neuroscience was the subject that interested and stimulated me the most. More specifically, it was by observing a patch clamp electrophysiological recording of a neuron triggering bursts of action potentials that I decided to go into neuroscience."
Why IINS?
"In 2007, I went to Bordeaux for the first time to participate in the "Escube" summer school organised by Christophe Mulle. It was a very enriching experience with quality science and an active scientific community! The following year, I decided to join Christophe’s team as a postdoctoral researcher. Then, in 2013, I obtained a research position at the CNRS. And, from 2017 to 2022, I did a mission and then a secondment in James Poulet’s laboratory. There I studied how in vivo cortical circuits encode sensory information and control behaviour. In 2022, I finally returned to Bordeaux to co-lead the "Synapses and neural circuits" team alongside Christophe. This was a great opportunity for me. Indeed, it allowed me to gain autonomy and to develop my own research. Moreover, this co-direction allowed me to benefit from the collaboration of an experienced and motivated scientist like Christophe."
Tell us about your research
"Currently, I am focusing to studying the cellular mechanisms and circuits underlying taste processing. So, by studying how cortical neurons respond to taste stimuli, I want to understand how the brain processes and integrates sensory information to generate appropriate behavioural responses. This research could have implications for understanding the neural basis of eating behaviour and related disorders."
Any advice for young researchers?
"First of all, choose and develop a research project that you are passionate about and that makes you happy! This will help you stay motivated and dynamic throughout your (long and sometimes difficult) research. Also, find a laboratory with a healthy and positive environment where you can develop personally and professionally."
How does our brain encode cold and warm? by Mario Carta
The cellular coding of temperature in the mammalian cortex.
M. Vestergaard, M. Carta, G. Güney & J. F. A. Poulet - Nature. 2023 Feb 8. doi: 10.1038/s41586-023-05705-5. Online ahead of print.
Warm, cool, these sensations are an integral part of our daily lives. Our ability to detect the temperature of the objects is essential to living. For almost a century, scientists have been trying to determine where in the brain the ability to detect temperatures is located. A study published in Nature reports the discovery of a 'thermal cortex' located in a posterior region of the insular cortex. This cortex can detect cool or warm temperatures.
When our body is in motion, our brain processes the information from our sensory organs. This processing then builds a conscious perception of the world. Much of this process takes place in the outer, folded layer of the brain, called the cortex. The cortex has a major role! It is the house of high brain functions, such as voluntary movement and consciousness.
The scientists started from a previous observation: neurons in the primary somatosensory cortex are active when the skin comes into contact with cool temperatures. They therefore expected that warm temperatures would also be encoded in this region. A test was therefore carried out on mice. To do this, they exposed the front paws of the mice to slight changes in temperature. They then used imaging techniques to find out which part of the brain reacted to these temperature changes. To their surprise, the scientists found that the primary somatosensory cortex did not respond to warming, but on closer inspection, the posterior insular cortex instead responded not only to cooling but also to warming. Using a two-photon microscope, the response of individual neurons in the posterior insular cortex was studied. The scientists discovered that there are cool-specific neurons, warm-specific neurons and neurons that respond to both warm and cool. It should be noted that 'warm' and 'cool' neurons react in very different ways. Indeed, "warm" neurons are sensitive to absolute temperature, while "cool" neurons are activated following a variation in temperature. In addition, the responses of the "cool" neurons are faster than the responses of the "warm" neurons. This indicates that there are potentially separate pathways for the perception of cool and warmth.
To prove conclusively that the insular cortex is involved in temperature perception, the scientists trained mice to respond to cool or warm temperatures. Using optogenetics they were able to temporarily deactivate the posterior insular cortex while delivering a thermal stimulus. In the end, the mice did not feel the thermal stimulus. However, when the scientists stopped deactivating the posterior insular cortex, they felt the stimulus again.
This discovery and the possibility of optically accessing the cortical representation of sensory processing in the insula opens up new avenues of research. First, to study the neural mechanisms of thermal perception, but also to extend similar research to other sensory systems represented in adjacent parts of the insular cortex (in particular the gustatory and visceral systems).
They speak about it too: INSB; CNRS; Bordeaux Neurocampus
Read the portrait of Mario Carta
Contact:
- Mario Carta, chercheur CNRS, mario.carta@u-bordeaux.fr
- James Poulet, professor and group leader, james.poulet@mdc-berlin.de
Christophe Mulle appointed "Chevalier de l’Ordre National du Merite"
Christophe Mulle is a research director at the CNRS and co-director of the "Synapse and neuronal circuits" team at IINS. A cellular neurobiologist, he specialises in glutamate receptors, in the electrophysiology of synaptic transmission and neuronal circuits. His research focuses on the plasticity of synaptic properties and neuronal circuits in the hippocampus, in the context of episodic memory encoding. Christophe Mulle also explores the mechanisms of synaptic dysfunction that occur in the context of Alzheimer's disease. At the same time, he is involved in a translational project to combat temporal lobe epilepsy using gene therapy, in close collaboration with Valerie Crepel. This has led to the creation of a start-up, Corlieve Therapeutics, of which he is scientific co-founder.
Christophe Mulle has invested a great deal of time in coordinating actions for the neuroscience research community in Bordeaux, as well as at a national and European level. For example, he has created the Bordeaux School of Neuroscience, which hosts the prestigious international Cajal training courses.
Christophe Mulle has been made a "Chevalier de l’Ordre du Merite" by the French Minister for Research. The award will be presented to him by Manuel Tunon de Lara, former President of the University of Bordeaux and himself a "Chevalier de l'Ordre du Merite", on Friday 2 June 2023.
Instituted by the General de Gaulle, "l’Ordre National du Merite" is the second national order after the Legion d'Honneur. Its purpose is to reward "distinguished merit" and encourage the country's driving forces.
Christophe Mulle has already won numerous awards. Nevertheless, all of them have a real meaning for the researcher.
What does this new award mean to you?
"It is an honour, of course, even if it was completely unexpected. It was Frederique Vidal, the former Minister for Research, who announced it to me in a letter in the middle of the Covid period. First of all, I looked into the award to find out what it was all about. In the end, of course, I am delighted!"
Why did you get it?
"I do not know who nominated me and why. Unlike a prize such as that the one from the Academie des Sciences, which rewarded the research I had carried out in the field of synapse biology, I suppose that this nomination bears witness to the role that I played in structuring neuroscience research in Bordeaux, and in promoting this discipline at both national and European level."
Any words for the scientific community?
"It is a real privilege to do the job we do. The role you can play in coordinating training and research structures is important and really satisfying! Nevertheless, I never lose sight of the fact that my job is to be a researcher. Driven by curiosity about how things work and what they are used for, it is an immense pleasure to come to the lab and discuss research issues with all my colleagues, the young and more established alike. And then, of course, to implement a wide range of projects. And at Bordeaux Neurocampus, a real bonus!"
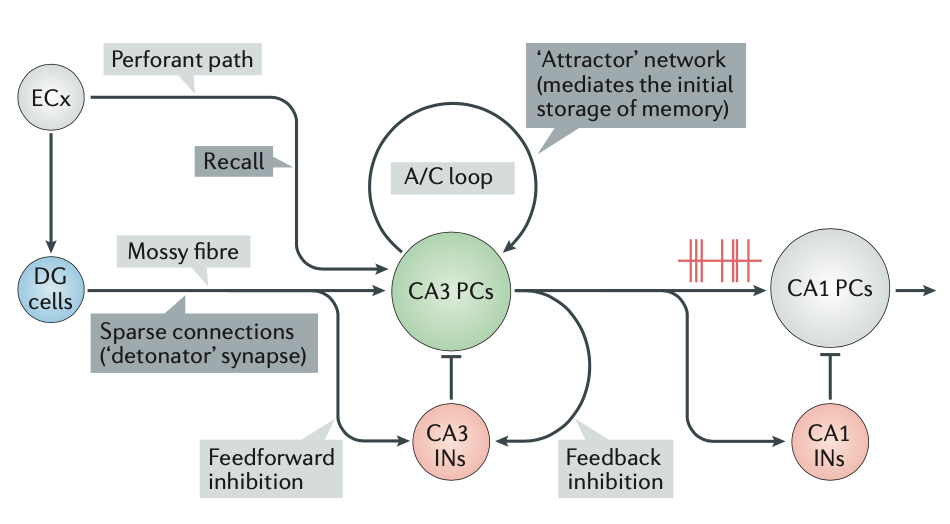
GluK2 Is a Target for Gene Therapy in Drug-Resistant Temporal Lobe Epilepsy
*Boileau, C., *Deforges, S., *Peret, A., Scavarda, D., Bartolomei, F., Giles, A., Partouche, N., Gautron, J., Viotti, J., Janowitz, H., Penchet, G., Marchal, C., Lagarde, S., Trebuchon, A., Villeneuve, N., Rumi, J., Marissal, T., Khazipov, R., Khalilov, I., Martineau, F., Maréchal, M., Lepine, A., Milh, M., Figarella-Branger, D., Dougy, E., Tong, S., Appay, R., Baudouin, S., Mercer, A., Smith, J.B., Danos, O., Porter, R., #Mulle, C., #Crépel, V., 2023. Ann Neurol. doi:10.1002/ana.26723, *co-first authors, # co-senior authors
Summary
The teams of Christophe Mulle (CNRS researcher and IINS Team Leader, University of Bordeaux) and Valerie Crepel (INMED, Inerm, Marseille) have demonstrated that localized injection of a viral vector targeting GluK2/GluK5 kainate receptors in the hippocampus is a highly promising gene therapy strategy for controlling seizure onset in drug-resistant epilepsy patients. Based on the gene therapy strategy described in the Annals of Neurology article, the Dutch company uniQure, which has acquired the start-up Corlieve Therapeutics - of which Valerie Crepel and Christophe Mulle were scientific co-founders - will launch a Phase I/IIa clinical trial in the USA to for the treatment of drug-resistant temporal lobe epilepsy.
Presentation of the article
This article is the fruit of a long-standing collaboration between Christophe Mulle and Valerie Crepel. It represents a key step in the development of a gene therapy approach for the treatment of temporal lobe epilepsy. Epilepsy, which consists in the synchronized, abnormal excitation of a group of neurons in the cerebral cortex, affects around 600,000 people in France. Temporal lobe epilepsy is the most common form of epilepsy in adults, with the affected area mainly in the hippocampus. Medical treatment, using antiepileptic drugs to normalize the hyperactivity of cortical circuits, is effective in 70% of cases. For drug-resistant forms, surgical resection is often the only option left. Against this backdrop, the two teams have developed a translational project targeting kainate-type glutamate receptors.
This project is based on the hypothesis that GluK2/GluK5-type kainate receptors localized ectopically at aberrant synapses formed by the sprouting of axons from dentate gyrus granule cells, act as detonators in triggering epileptic discharges in the hippocampus. In 2014, an initial study by these researchers demonstrated that the genetic deletion of grik2, the gene encoding the GluK2 subunit of the kainate-type glutamate receptors, or pharmacological inhibition of GluK2/GluK5 receptors, led to a reduction in the number of spontaneous and recurrent seizures observed in a mouse model of temporal lobe epilepsy. A patent had been filed indicating GluK2/GluK5 as a potential target for treating temporal lobe epilepsy.
In the "Annals of Neurology" article, the teams of Valerie Crepel and Christophe Mulle (and in his team the work carried out mainly by Severine Deforges, IR CNRS), chose to develop a strategy based on interfering RNAs to reduce grik2 gene expression locally in the hippocampus. The article describes the strategy for selecting interfering RNA sequences in cell models, and the production of a viral vector that enables expression of an anti-grik2 microRNA in hippocampal neurons. This viral vector was injected in vivo into epileptic mice. The article demonstrates the efficacy of this viral vector in decreasing grik2 expression and GluK2 levels in the hippocampus, and ultimately in reducing epileptic activity in mice in vivo. Moreover, administration of the viral vector to organotypic hippocampal slices obtained from surgically resected epileptic patients, led to the suppression of epileptiform discharges recorded in these slices.
In conclusion, localized injection of a viral vector targeting grik2 expression in the hippocampus represents a highly promising gene therapy strategy for controlling seizures in drug-resistant epilepsy patients.
The work described in this article goes hand in hand with an industrial drug development strategy that Valérie Crépel and Christophe Mulle have been pursuing for several years, with the support of Aquitaine Science Transfert, Inserm Transfer and the Kurma Partners investment fund. Valerie Crepel and Christophe Mulle were the scientific co-founders of start-up Corlieve Therapeutics in October 2019, a company that was acquired in July 2021 by uniQure, a Dutch company specializing in the development of gene therapy for neurological diseases.
In September 2023, in parallel with the publication of the article in Annals of Neurology, uniQure issued a press release (see attached pdf), announcing that “the U.S. Food and Drug Administration (FDA) has cleared the Investigational New Drug (IND) application for AMT-260, the Company’s gene therapy candidate for refractory mesial temporal lobe epilepsy (MTLE).” “AMT-260 comprises an AAV9 vector that locally delivers two engineered miRNAs designed to degrade the GRIK2 gene and suppress the aberrant expression of glutamate receptor subtype GLUK2 that is believed to trigger seizures in patients with refractory MTLE.”. Accordingly, following the gene therapy strategy described in the Annals of Neurology article, uniQure will launch a Phase I/IIa human clinical trial to be conducted in the US in early 2024.

Selected Publications
MORE
MORE
MORE
MORE
MORE
MORE
MORE
MORE
MORE
MORE
MORE
MORE
MORE
MORE
MORE
MORE
MORE
MORE
MORE
MORE
MORE
MORE
MORE
MORE
Members
« Researcher »
| AMEDEE Thierry | Researcher | thierry.amedee@u-bordeaux.fr | +33533514757 |  |
| CARTA Mario | Researcher | mario.carta@u-bordeaux.fr | +33533514759 |  |
| MULLE Christophe | Researcher | christophe.mulle@u-bordeaux.fr | +33533514716 |  |
« Technical Staff »
| DEFORGES Severine | Technical staff | severine.deforges@u-bordeaux.fr | +33533514780 |  |
| GROSJEAN Noelle | Technical staff | noelle.grosjean@u-bordeaux.fr | +33533514780 |  |
| PREVOST Lucie | Technical staff | lucie.prevost@u-bordeaux.fr | +33533514700 |
« Postdoc »
| MOREIRA DE SA Ana | Postdoc | ana.moreira-de-sa@u-bordeaux.fr | +33533514769 |  |
| MUTTATHUKUNNEL Paola | Postdoc | paola.muttathukunnel@u-bordeaux.fr | +33533514700 |  |
« PhD student »
| BARBA VILA Olga | PhD student | olga.vila@u-bordeaux.fr | +33533514700 |  |
| ERHARDT Anaël | PhD student | anael.erhardt@etu.u-bordeaux.fr | +33533514780 |  |
| LECOMTE Simon | PhD student | simon.lecomte@u-bordeaux.fr | +33533514700 |  |
| MIRA Bruna | PhD student | bruna.mira@u-bordeaux.fr | +33533514700 | |
| TORTOCHOT-MEGNE FOTSO Mathilde | PhD student | mathilde.tortochot-megne@u-bordeaux.fr | +33533514700 |
« Student »
| COLLETTE Margot | Student | margot.collette@u-bordeaux.fr | +33533514700 |