Dynamic Organization and Function of Synapses
Team Leader : Daniel Choquet

"Progress in science depends on new techniques, new discoveries and new ideas, probably in that order." Sydney Brenner
Daniel Choquet is a research director at the CNRS. He obtained an engineering degree from Ecole Centrale (Paris, France) in 1984 and completed his PhD in the lab of Henri Korn at the Pasteur Institute (Paris), studying ion channels in lymphocytes. He did a post-doctoral/sabbatical at Duke University (North Carolina, USA) in the laboratory of Michael Sheetz where he studied the regulation of integrin-cytoskeletal linkage by force. He setup his group in Bordeaux (France) at the Institute for Neuroscience and launched an interdisciplinary program on the combination of physiology, cell and chemical biology and high resolution imaging to study the functional role of the dynamic organization and trafficking of neurotransmitter receptors in synaptic transmission. He is now heading the Institute for Interdisciplinary Neuroscience and the Bordeaux Imaging Center core facility. He is also the director of the center of excellence BRAIN. He is a Member of the French Science Academy and has been awarded three consecutive ERC advanced grants. BioSketch (PDF).
You can check here the Personal page of Daniel Choquet.
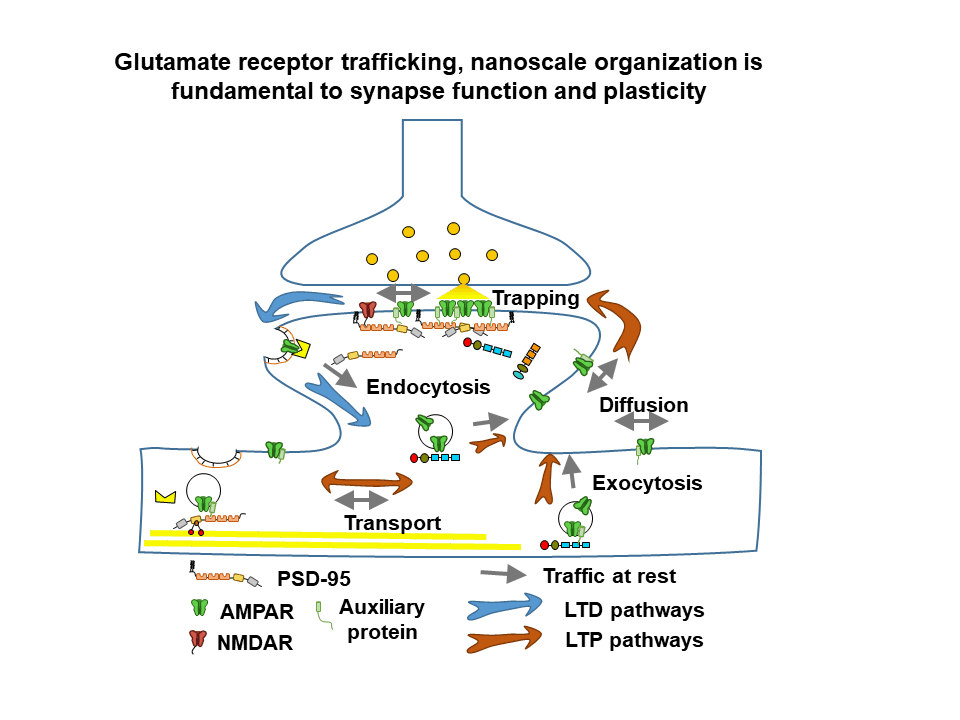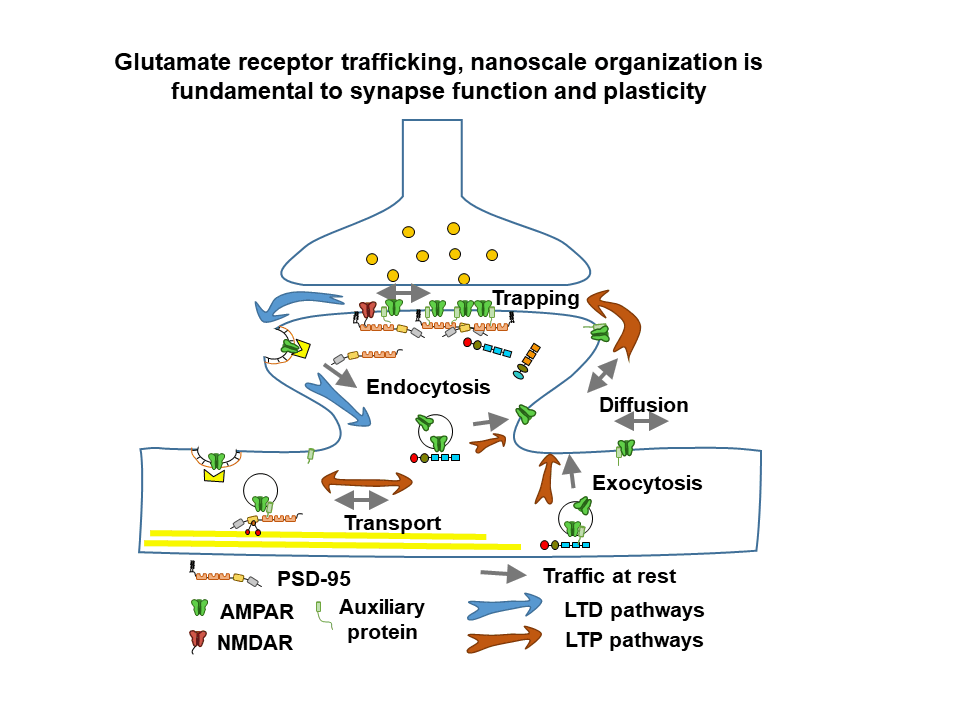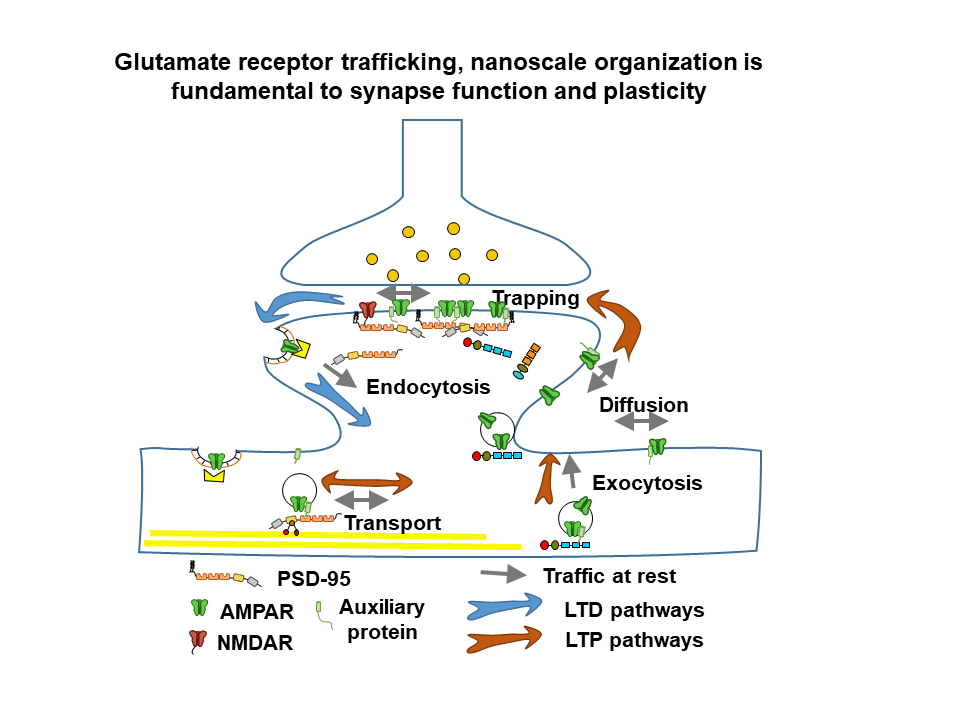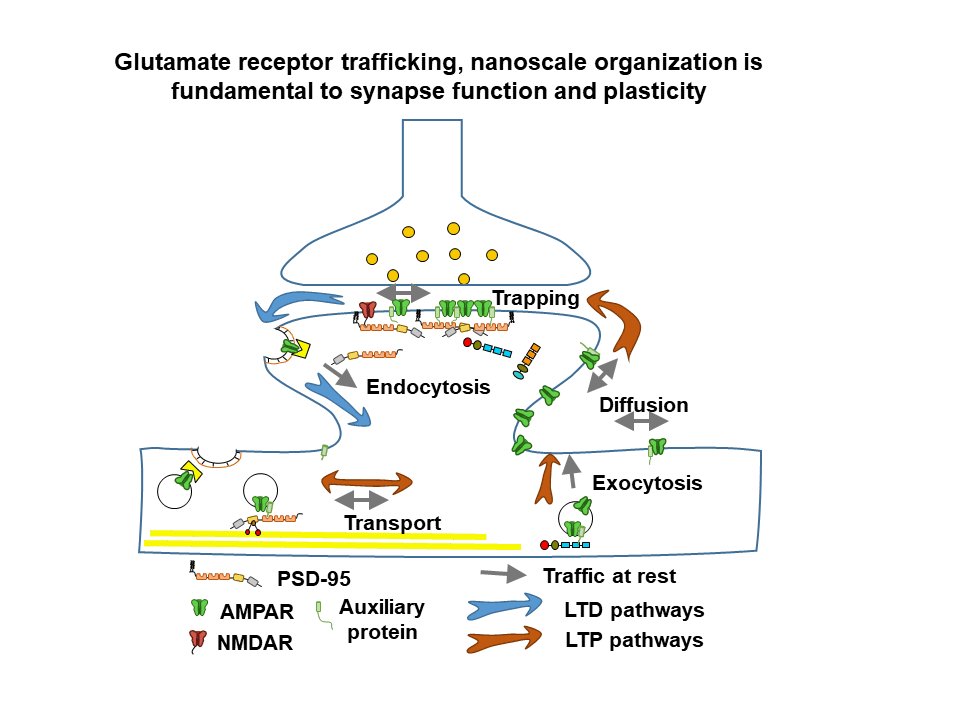
General objective

The team develops several research topics, combining neuroscience, physics and chemistry in order to unravel the dynamics and nanoscale organization of multimolecular receptor complexes and their functional rôle in glutamatergic synaptic transmission in health and disease. Recently, the team has engaged in a major program to analyze and understand the interplay between AMPA type glutamate receptor nanoscale dynamics, synaptic plasticity and memory formation in the healthy and diseased brain. Our latest developments are a major thrust in combining molecular and physiological studies as well as analyzing neurodevelopmental disorders related to synapse dysfunction.
Project leaders (Click to reach subprojects)
Research Projects
Team Project Summary
MORE

Cytoskeleton and membrane interplay in neuronal and astrocytic organization and functions (Anna Brachet).
MORE

Chemical Biology and Protein Engineering (Matthieu Sainlos)
MORE

Impact of auxiliary proteins on AMPAR transport, trafficking and physiology (Françoise Coussen).
MORE

nanoscale excitatory synaptic physiology (Eric Hosy).
MORE

Contribution of AMPAR surface trafficking to Short and long term synaptic plasticity (Daniel Choquet)
MORE

Synapse dysfunction in neurodevelopmental disorders (Eric Hosy, Daniel Choquet)
MORE
Partnerships
MOREExpertise
News
Multicolor Spectrin labeling (ML Jobin)
This is a rat hippocampal neuron in culture stained with a series of markers

Nature Neuroscience Review - Choquet D., Sainlos M. and Sibarita J.B.
We review the latest developments for labelling and functionalizing proteins with small localization and functionalized reporters. We present how these molecular tools are combined with the development of a wide variety of imaging methods that break either the diffraction barrier or the tissue penetration depth limits. We put these developments in perspective to emphasize how they will enable step changes in our understanding of the brain.
MORE
Daniel Choquet: prix 2022 de l’Academie nationale des sciences, belles lettres et arts de Bordeaux
Academie nationale des sciences, belles lettres et arts de Bordeaux aims to help develop the ideas, work and research of Academicians. Each year, it rewards in particular personalities for their work or their research or for all of their work in the field of science, literature or the arts.
Thus, in 2022, it was Daniel Choquet, CNRS research director, who was awarded "le grand prix 2022 de l’Academie nationale des sciences, belles lettres et arts de Bordeaux"! Indeed, he achieved this distinction thanks to his main scientific achievement: the discovery that neurotransmitter receptors are in constant motion in the neuronal membrane and that the regulation of this traffic profoundly regulates synaptic transmission. Daniel Choquet will receive his award on March 28 from Pierre Hurmic, Mayor of Bordeaux.
Learn more about: Le grand prix 2022 de l’Academie nationale des sciences, belles lettres et arts de Bordeaux
They talk about it too (articles in French): INSB and Bordeaux Neurocampus
Do you want to know more? Nolwenn Cloarec, communication officer: nolwenn.cloarec@u-bordeaux.fr
Regulation of different phases of AMPA receptor intracellular transport by 4.1N and SAP97, eLife
Caroline Bonnet1, Justine Charpentier1, Natacha Retailleau1, Daniel Choquet1,2, Françoise Coussen1*
1University of Bordeaux, CNRS, Interdisciplinary Institute for Neuroscience, Bordeaux, France; 2Bordeaux Imaging Center, Bordeaux, France
eLife. 2023-04-20
DOI: https://doi.org/10.7554/eLife.85609
Françoise Coussen, Director of research at the CNRS at IINS worked on AMPAR intracellular transport and directed this work helped by Daniel Choquet. Caroline Bonnet, PhD student, performed the experiments helped by Justine Charpentier who performed all biochemistry experiments and by Natacha Retailleau (molecular biology).
Find the explanations of the scientists of this publication
Identification of the molecular mechanisms regulating the intracellular transport of glutamate receptors: a new pathway for controlling synaptic plasticity
"The modulation of the efficiency of synaptic transmission between neurons is one of the fundamental processes of memory and learning phenomena. This regulation of the strength of synaptic transmission is largely driven by changes in the number of receptors present at the synapse. In this work, the researchers identify a new mechanism for controlling the establishment of receptors at the level of the synapse through the control of their intracellular transport.
Neurotransmitter receptors, and in particular glutamate receptors, are concentrated in synapses in front of neurotransmitter release sites. However, in the process of their biogenesis, these receptors are synthesized at the level of the endoplasmic reticulum, most of the time several hundred microns from the synapses. They must therefore be transported to the synapses. Our previous work had made possible to visualize for the first time the intracellular transport of AMPA-type glutamate receptors, responsible for the majority of the rapid excitatory transmission between neurons. These receptors are transported rapidly (1-2 microns per second) in vesicles circulating on the microtubules using molecular motors. We observed that, surprisingly, this transport was strongly regulated by neuronal activity.
In this new work, we have identified the molecular mechanisms responsible for these regulations. The cytosolic C-terminal domain of the AMPAR GluA1 subunit is specifically associated with two proteins, 4.1 N and SAP97. We analyzed how interactions between GluA1 and 4.1N or SAP97 regulate GluA1 transport and its exocytosis under basal conditions and after induction of synaptic plasticity (LTP). Our results identify differential roles of 4.1N and SAP97 in controlling the different phases of transport and membrane integration of GluA1.
This work opens new perspectives in understanding the molecular mechanisms that control the establishment and maintenance of glutamate receptors at the synapse during synaptic plasticity."
Daniel Choquet is a member of the 2023 Fulbright France
Created in 1946 at the proposal of the eponymous US senator, Fulbright is an international academic mobility program of the US government. The aim of the program is to promote educational and cultural exchanges between France and the United States.
Since its launch, 21,244 students from both countries have benefited from the program:
- 12,294 French students have gone to the United States of America,
- 8,950 Americans have come to France.
Daniel Choquet (director of research at the CNRS, director of IINS, the BIC, the cluster of excellence BRAIN_2030 and team leader at IINS) has just been selected as a laureate of the Fulbright France program. He will be spending a 7 month sabbatical stay in Denver in 2024.
Why did you apply to the Fulbright France Program?
“I was getting organized to perform a 7 month sabbatical in Denver in 2022, in the lab of Pr. Mark Dell’Acqua. Given the cost of living in the US, I needed additional funding to support my stay. The Fulbright organization is a very prestigious academic mobility program of the US government and it was thus natural for me to apply, particularly given that there is a specific partnership with our region Nouvelle-Aquitaine.”
What does it mean to you?
“Well, it does mean a lot. First, in searching the Fulbright alumni file, I found out that both my parents were fulbrighters in the 50s while they did post-docs in Princeton, my mother with Albert Einstein. Second, I realized when I obtained this fellowship that when you enter a new family, the family of Fulbrighters, beyond the money, you enter a support network all over the US that will be both very interesting and valuable.”
Will you take this opportunity to discover or develop a research topic?
“Absolutely, the Department of Pharmacology at the University of Colorado in Denver that I’ll join hosts a large number of high profile neurobiologists that develop orginal cell biology approaches.
We will join our forces with Mark Dell’Acqua and Matthew Kennedy to develop new methods and approaches to control receptor trafficking and visualization.”
What do you think this program will bring you, both professionally and personally?
“In addition to the funding that will help me live there, being a Fulbright fellow in the US is really recognized and will help me develop my network and meet many academics in various disciplines. Fulbright fellow also have a mission to be ambassadors of their country, and I certainly intend to introduce to colleagues to some French cuisine specialties!”
#IMadteIt: Marie-Lise Jobin, a post-doctoral researcher starting out as an associate professor
Marie-Lise Jobin is currently a post-doctoral researcher in Daniel Choquet’s "Dynamic organization and function of synapses" team. Interested in the role of the cytoskeleton in the function and structure of dendritic spines, the scientist is supervised by Anna Brachet. Marie-Lise recently obtained a permanent teaching-research position at the Institut National Polytechnique de Bordeaux (Bordeaux INP). Here, she looks back on her career.
What is your background?
"I studied biology and structural biochemistry at the University of Bordeaux. During my Master’s and PhD, I worked in many different laboratories. First, I spent three months working at the University of Guelph in Ontario, Canada. Then, for my PhD, I joined the Institute of Chemistry and Biology of Membranes and Nano-objects (CBMN) in Pessac. On completion of my studies, I spent a year as a post-doctoral fellow at the Nutrition and Integrative Neurobiology Laboratory (Nutrineuro) in Bordeaux. I then spent over three years at the University of Würzburg in Bavaria, Germany. And for the last four years I’ve been at IINS. My post-doctoral adventure will finally come to an end at the end in September 2023, and I’ll be taking up a position as an associate professor in September!"
Why did you join IINS?
"Generally speaking, I’ve always been interested in biomolecular interactions involving cell membranes. I first worked on synthetic membrane models. However, I’ve always wanted to study more physiological systems. So the neuroscience field allows me to work with systems closer to physiology, and to use my highly interdisciplinary skills. [...] Anna Brachet’s field of research on the role of cytoskeletal proteins, in particular spectrins, and their partners, in the regulation, function and mechano-transduction of dendritic spines fit perfectly my research interests. Moreover, IINS is considered one of the most recognized institutes in the field of neuroscience. Above all, IINS is part of a synergistic system, with close relations to the Bordeaux Imaging Center (BIC). It was a unique opportunity to develop my skills in cutting-edge microscopy techniques and gain access to considerable knowledge in this field!"
Can you tell us about your research?
"I’m trying to understand the role of cytoskeletal proteins, beta spectrins, in neurons, and more specifically in dendritic spines, which are the seat of neuronal transmission. My results showed that beta spectrins are co-organised on a nanoscopic scale in dendritic spines and are particularly important for the stability of these structures. Their membrane or cytoskeleton partners are all the more important in maintaining and stabilising these spectrins under the membrane and influence their nano-organisation."
As a woman in science, what difficulties have you encountered?
"My biggest difficulty has always been a lack of self-confidence. But throughout my career I’ve been lucky enough to meet scientists, men and women who have had faith in me, and who have always supported and encouraged me along the way."
Why do women need to be more recognised in the scientific community?
"It seems quite logical to me that gender diversity, or diversity of any kind, leads to better, more creative and innovative science. I can see that things are changing, slowly but surely, in the right direction, and I’m optimistic for the future!"
Any advice for young researcher?
"I’m proud to have got where I am, to have succeeded in obtaining a permanent position in academia, and to have stayed the course despite many years of not knowing what would happen and never giving up. You have to persevere. It’s often the fear of failing that stops us from moving forward, so don’t hesitate, dare to take the plunge and, above all, trust yourself."
2 days of exciting science on synapse biology around the PhD defense of Agata Nowacka
Monday 6th November 2023
Workshop: Virtual Presynaptic Terminal
We invite you to a workshop where a collaborative team from University College London (UCL) and the University of Warwick will introduce a computational modelling framework that enables in silico exploration of the mechanisms of Ca²⁺-driven neurotransmitter release in different synapses.
Morning session 9:00 – 12:30
• Kirill Volynski: Introduction to the modelling framework with examples linking the model and experimental data.
• Yulia Timofeeva: 3D modelling of presynaptic Ca2+ dynamics, covering Ca2+ influx, buffering, and diffusion.
• Chris Norman: Modelling Ca 2+ activation of vesicular release and short-term synaptic plasticity.
• Round table: model expansion across the synaptic cleft.
Afternoon session 14:00 – 17:00
• Yulia Timofeeva and Chris Norman: hands-on training for application of the virtual presynaptic terminal model. Contact the presenters to book a one-to-one training session.
Registration before October 20: https://evento.renater.fr/survey/workshop-virtual-presynaptic-terminal-iyqqwh9v
Tuesday 7th November 2023
Seminar Molecular and cellular mechanisms of synapse physiology
Venue: Centre Broca Nouvelle-Aquitaine (Amphitheater Broca)
Program
9h30 - Maria Passafaro "Unraveling PCDH19-related pathogenic mechanisms in Developmental and Epileptic Encephalopathy 9 (DEE9)"
10h10 - Andrea Barberis 'Spatial determinants for the interactions of glutamatergic and GABAergic synapses in dendrites of hippocampal pyramidal neurons"
10h50 - Kirill Volynski "Synergistic regulation of neurotransmitter release by different synaptotagmin isoforms"
11h30 - Aude Panatier "Astrocytic EphB receptors control NMDAR functions and memory"
14h - PhD defense of Agata Nowacka "Differential contributions of pre- and postsynaptic components in tuning high-frequency short-term synaptic plasticity"
Contact
Kirill Volynski (k.volynski@ucl.ac.uk), UCL Queen Square Institute of Neurology
Chris Norman (c.norman@ucl.ac.uk), UCL Queen Square Institute of Neurology
Yulia Timofeeva (y.timofeeva@warwick.ac.uk), Department of Computer Science, University of Warwick
PhD Project Overview: AMPA Receptor Dynamics in Synaptic Plasticity
Project Title: Unraveling AMPA Receptor Dynamics in Synaptic Plasticity Using Cutting-edge Imaging and Electrophysiology
Introduction
Synapses, the communication hubs of the brain, rely on their molecular composition and organization for effective information processing. AMPA receptors (AMPARs) are crucial in this process as they mediate fast excitatory synaptic transmission, which is essential for learning and memory. This project aims to explore how the mobility and nanoscale organization of AMPARs influence synaptic plasticity and information processing.
Research Objectives
- Investigate AMPAR Trafficking:
- Understand how AMPAR mobility and nanoscale organization control synaptic plasticity.
- Study the interaction of AMPARs with auxiliary proteins like TARP, CNIH, and Shisas.
- Develop and Apply Advanced Techniques:
- Combine electrophysiology on brain slices with light sheet imaging, optogenetics, and cell biology.
- Utilize innovative tools to control receptor mobility and subunit composition.
- Implement High-Resolution Imaging:
- Enhance Light Sheet Fluorescence Microscopy (LSFM) and Lattice Light Sheet Microscopy (LLSM) with adaptive optics for deep tissue imaging.
- Integrate Single Molecule Localization Microscopy (SMLM) techniques like U-PAINT and DNA-PAINT for nanometer-scale resolution imaging of AMPAR subunits.
Methodology
- Electrophysiology: Perform recordings on brain slices to analyze synaptic activity and plasticity.
- Light Sheet Imaging: Use LSFM and LLSM to visualize synapses in thick samples with minimal phototoxicity.
- Optogenetics: Control receptor dynamics using light-sensitive proteins to manipulate signaling pathways.
- SMLM Techniques:
- U-PAINT: Track individual AMPAR movements in rat hippocampal cultures.
- DNA-PAINT: Achieve high-resolution imaging of GluA1 and GluA2 subunits in organotypic brain slices.
Innovations and Expected Outcomes
- Adaptive Optics Integration: Correct optical aberrations in LLSM for clearer imaging.
- Advanced Marking Techniques: Develop robust methods for deep SMLM imaging in brain slices.
- Intracellular Signaling Control: Integrate photoswitchable kinases and phosphatases to study receptor dynamics.
Student Opportunities
- Hands-on Experience: Gain practical skills in electrophysiology, advanced microscopy, and molecular biology.
- Cutting-edge Research: Contribute to groundbreaking studies on synaptic plasticity and receptor dynamics.
- Collaborative Environment: Work with a team of experts in neuroscience and cutting-edge imaging technologies.
Join us in exploring the intricate world of synaptic transmission and plasticity, and make a significant impact on our understanding of brain function and diseases.
To apply, please send complete CV, letter of motivation and up to two letters of recommendations: daniel.choquet@u-bordeaux.fr
Selected Publications
Display the article MORE
Display the article MORE
Display the article MORE
Display the article MORE
Display the article MORE
MORE
Display the article MORE
Display the article MORE
Display the article MORE
Display the article MORE
Display the article MORE
Display the article MORE
Display the article MORE
Display the article MORE
Display the article MORE
Display the article MORE
Display the article MORE
Members
« Researcher »
| BRACHET Anna | Researcher | anna.brachet@u-bordeaux.fr | +33533514732 |  |
| CHOQUET Daniel | Researcher | daniel.choquet@u-bordeaux.fr | +33533514715 |  |
| COUSSEN Francoise | Researcher | francoise.coussen-choquet@u-bordeaux.fr | +33533514734 |  |
| HOSY Eric | Researcher | eric.hosy@u-bordeaux.fr | +33533514730 |  |
| SAINLOS Matthieu | Researcher | matthieu.sainlos@u-bordeaux.fr | +33533514731 |  |
« Technical Staff »
| BADRUNA Louise | Technical staff | louise.badruna@u-bordeaux.fr | +33533514700 | |
| BELZANNE Pauline | Technical staff | pauline.belzanne@u-bordeaux.fr | +33533514700 |  |
| BREILLAT Christelle | Technical staff | christelle.breillat@u-bordeaux.fr | +33533514731 | |
| CHEVRIER Nicolas | Technical staff | nicolas.chevrier@u-bordeaux.fr | +33533514733 |  |
| Daburon Sophie | Technical staff | sophie.daburon@u-bordeaux.fr | +33533514732 |  |
« Postdoc »
| CORNEBOIS Anaïs | Postdoc | anais.cornebois@u-bordeaux.fr | +33533514700 | |
| DOMART Florelle | Postdoc | florelle.domart@u-bordeaux.fr | +33533514700 |  |
| IGNACZ Attila | Postdoc | attila.ignacz@u-bordeaux.fr | +33533514700 |  |
| STAJANO Daniele | Postdoc | daniele.stajano@u-bordeaux.fr | +33533514700 |
« PhD student »
| CHANDRA Meera | PhD student | meera.meera-chandra@u-bordeaux.fr | +33533514700 | |
| CHARBONNIER Tatiana | PhD student | tatiana.charbonnier@u-bordeaux.fr | +33533514700 |  |
| CUENOT Chloé | PhD student | chloe.cuenot@u-bordeaux.fr | +33533514700 |  |
| DARRIBERE Manon | PhD student | manon.darribere@u-bordeaux.fr | +33533514700 |  |
| GALIZIA Chiara | PhD student | chiara.galizia@u-bordeaux.fr | +33533514700 | |
| LEVAL Léa | PhD student | lea.leval@u-bordeaux.fr | +33533514700 | |
| PESSOA Emma | PhD student | emma.pessoa@u-bordeaux.fr | +33533514700 | |
| SARZYNSKI Léa | PhD student | lea.sarzynski@u-bordeaux.fr | +33533514700 |  |
| VILLICANA-MUÑOZ Viviana | PhD student | viviana.villicana-munoz@u-bordeaux.fr | +33533514700 |  |
« Alumni & Guests »
Former group members, follow-up last known position
- Aren borgdorff - 1997-2000, Industry
- Arnauld Sergé - 1997-2001, Assistant professor Marseille
- Marianne Renner - 2004-2006, Professor, Paris
- Caroline Dequidt - 2004-2007, Industry
- Cécile Bats - 2004-2007, Post-doc, London
- Martin Heine - 2003-2007, Junior group leader, Magdeburg
- Enrica Petrini - 2005-2008, Post-doc, Genoa
- Cezar Tigaret - 2006-2009, Lecturer, Bristol
- Helge Ewers - 2007-2009, Group leader, Berlin
- Arnaud Frouin - 2007-2010, Labmanager, NYC
- Leandro Royer - 2009-2012, Post-doc, Boston
- Patricio Opazo - 2008-2013, Group leader, Brisbane
- Jary Delgado - 2009-2013, Post-doc, Chicago
- Deepak Nair - 2009-2013, Group leader, Bengalor
- Damien Jullié - 2009-2013, Post-doc, San Francisco
- Audrey Constal - 2010-2013, Teacher
- Axel Athane - 2011-2013, Industry
- Dolors Grillo, 2011-2013, Post-doc, Spain
- Anne-Sophie Hafner - 2010-2014, Post-doc, Frankfurt
- Isabelle Gautherau - 2012-2014, Industry
- Amandine Philippat - 2013-2015, Industry
- Andrew Penn - 2010-2015, Junior group leader, Sussex
- Hongyu Zhang - 2010-2015, Post-doc, University of Bergen
- Estelle Toulme - 2013-2015, Post-doc, Berlin
- Jennifer Petersen - 2009-2016, Post-doc, NIH
- Yulia Krapivkina - 2013-2016, Industry
- Ngoc Van Thi Nhu - 2013-2016, Engineer, Montpellier
- Emilie Hangen - 2014-2017
- Ségolène Antoine - 2015-2017, French Industry
- Sara Crespillo - 2015-2017, Post-doc, Cambridge
- Célia Michel - 2014-2017, French Industry
- Benjamin Compans - 2014-2018, Post-doc, London
- Lucile Pret - 2018, Engineer, London
- Julia Goncalves – 2015 – 2018, Engineer, Bordeaux Neurocampus
- Murielle Fevre - 2017-2018
- Camille Genuer - 2017-2018, Engineer, French Industry
- Carla Montecinos - 2013-2018, post-doc Bordeaux
- Charlotte Rimbault – 2014-2019, Post-doc, Copenhagen
- Magalie Martineau – 2015-2019, Post-doc, Paris
- Léa Claverie – 2015 - 2019, UK
- Florelle Domart - 2016-2019, Post-doc Goettingen
- David Perrais - 2011-2020, Group Leader
- Sylvia Sposini - 2018-2020, Post-doc, Bordeaux
- Lou Bouit - 2018-2020, Engineer, Bordeaux
- Côme Camus - 2018-2021, Medical Intern, Bordeaux
- Caroline Bonnet - 2017-2021
- Inès Gonzalès-Calvo - 2019-2021
- Valeria Pecoraro - 2019-2021
- Marie-Lise Jobin, 2019-2023 Maîtresse de Conférences (INP, Bordeaux)
Guests
- Raffaella Adami, Pisa - January, June 2001
- Andres Villu Maricq, Utah - November 2003
- Radhika Reddy, Worley lab - June 2003
- Michael Ehlers, Duke University - March, September 2006
- Renatto Frischknecht, Gundelfinger lab - April 2006, September 2006
- Laura Andreae, Fine lab - April 2006, November 2006
- Anna Carbone, Plested Lab - January, April 2012
- Beulah Leitch, Otago University - October 2013
- Andrew Plested, FMP Berlin - October 2015, February 2016
- Nikolaj Riis Christensen, Copenhagen University - June-December 2017
- Anne Brunet, Stanford University - April-July 2019
- Pin Wu Liu, Kyoto University Graduate School of Medicine - January 2020
- Boram Lee, Johannes W. Hell UCDavis - January-June 2020




